ENJOY AN EXTRA 10% OFF YOUR FIRST ORDER
When you subscribe to ProBioraHealth.com emails
by Jennifer Chiang, DDS

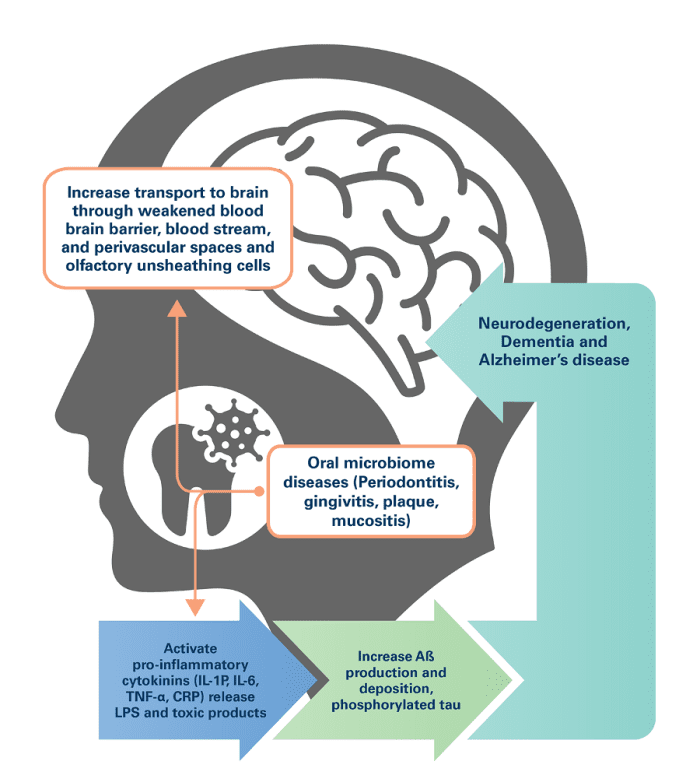
The oral microbiome is the collection of all microorganisms in the oral cavity including bacteria, funghi, and viruses. It is the second-largest and diverse microbiota after the gastrointestinal tract.1 Bacterial communities form biofilm (dental plaque) on teeth and in oral, gingival, and mucosal tissue. A high pathogenic load can disrupt the oral microbiome, causing inflammation and dysregulation of the immune system response. While bacterial overgrowth and chronic inflammation can lead to a variety of local diseases like periodontitis and dental caries, translocation of pathogenic bacteria into the bloodstream can cause systemic disease in other organs including the heart, liver, and brain.2 Bacteria that originate from the oral microbiome have been implicated in many systemic illnesses such as cardiovascular disease, metabolic syndrome, respiratory infections, and cognitive decline, with the common link between them being inflammation. Increasing evidence suggests that there is a relationship between oral health, inflammation, and neurodegenerative conditions such as Alzheimer’s disease (AD).
A recent CDC report found that 47% of adults aged 30 years and older (approximately 65 million adults) have some form of periodontal disease: 30.0% with moderate periodontitis, 8.5% with severe periodontitis, and 8.7% with mild periodontitis.3-4 Periodontitis is a chronic inflammatory disease affecting tissues that surround and support the teeth. One of the most important etiologies of periodontitis is Porphyromonas gingivalis, a keystone gram negative bacterial pathogen.5 Keystone pathogens can orchestrate inflammatory disease by remodeling a normally benign microbiota leading to an imbalance between normal and pathogenic microbiota (dysbiosis). 6-7 Altered microbiota in the oral cavity can spread via the lymphatic and vascular systems, infecting tissue in the brain and indirectly contributing to inflammatory pathways through immune system activation.8 P. gingivalis can enter the bloodstream and perivascular spaces when localized tissue trauma occurs from brushing, flossing, and chewing, or in cases of gingival bleeding or bacterial permeation of weakened periodontal tissue.
In brain tissue, oral bacteria can cause neuroinflammation and damage to neuronal cells, which, over time, contributes to cognitive decline and neurodegenerative diseases like AD. One recent study found that individuals with Alzheimer’s disease had different oral microbiome pathogens compared to those without disease.9 Specifically, they had an increased abundance of bacteria associated with periodontal disease, such as Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. Findings suggest that chronic periodontal infection with these bacteria produce toxins that can damage neurons in the brain and may contribute to neurodegenerative processes and thus may be lifelong risk factors for AD development.10-11 In vivo, P. gingivalis secretes cysteine proteases called gingipains that damage neuronal cells. Immunoreactivity to gingipains is consistent with increased neuroinflammation in AD-afflicted brains as compared to those without, and colonization of P. gingivalis has been documented post-mortem in both human and animal brain studies.12-13 Other studies have found that oral health measures, such as brushing and flossing, are associated with a reduced risk of cognitive decline and Alzheimer’s disease.14 Additionally, the use of antibiotics that target oral bacteria has been shown to improve cognitive function in individuals with Alzheimer’s disease.15 While the exact mechanisms linking the oral microbiome and Alzheimer’s disease are still being investigated, it is thought that oral bacteria may enter the bloodstream through bleeding gums and travel to the brain, where they can contribute to the development of Alzheimer’s disease by promoting inflammation and the formation of amyloid plaques.
Nutrition is an important consideration for patients experiencing periodontal diseases as certain food sources have anti-inflammatory, anti-oxidative, and demonstrated antibacterial properties against P. gingivalis, including green tea, curcumin, cranberries, pomegranate, mango, resveratrol from grapes and wine, and rice extracts.16- 17 These polyphenols are also noted for inhibiting gingipain activity in the brain and are emerging as potential therapeutic treatments to mitigate cell damage and disease progression.18-19
Much like the gut microbiome, the therapeutic use of probiotics has shown effectiveness in maintaining microbial balance in the mouth.20 Research has demonstrated that oral probiotics have the effects of promoting microbiota diversity and reducing plaque accumulation similar to manual disruption of the biofilm through brushing and flossing.21-22 Several bacterial strains have demonstrated inhibitory effects on pathogen growth, the formation of oral biofilms, and the disruption of pre-formed biofilms, including Lactobacillus, Bifidobacterium, Lactococcus, and Streptococcus.23-24 A 2021 study found that Streptococcus salivarius is able to adhere to gingival fibroblasts and inhibit inflammatory cytokine IL-6 and IL-8 production in the presence of anaerobic bacteria such as P. gingivalis,25 Bifidobacterium and Lactobacillus were also shown to improve the clinical indicators of periodontitis such as attachment loss and pocket depth and bleeding on probing.26 In addition to their efficacy as antimicrobial agents, the use of these commensal bacteria may also support the integrity of oral tissue and, in turn, is likely to decrease the incidence of bacterial translocation.
A small 2018 double-blind placebo-controlled randomized clinical trial of middle-aged adults tested the total cultivable bacteria of the mouth before and after mechanical plaque removal against treatment with an orally administered probiotic sachet of Lactobacillus rhamnosus. At the six-and nine-month time points, bacterial cultures in both groups had decreased, with no significant difference in efficacy between the two modalities.27 Furthermore, other studies suggest that oral probiotics as an adjunctive to daily hygiene habits yield the lowest concentrations of overall pathogenic bacteria.28-29
Identifying the root causes of a patient’s oral dysbiosis may help to inform the most effective treatment strategy to optimize both mouth and brain health. Overall, while the relationship between the oral microbiome and Alzheimer’s disease is not yet fully understood, there is growing evidence to suggest that maintaining good oral health may be important for reducing the risk of cognitive decline and Alzheimer’s disease. Thus, the same healthy habits that we encourage to improve oral health may help minimize risk factors associated with Alzheimer’s disease: flossing, brushing, polyphenol rich foods, and oral probiotics. ProBiora Health® probiotics for the mouth crowd out bad bacteria responsible for the body’s inflammatory response that can lead to disease.
Join our network of hundreds of professional offices and learn more about our proprietary probiotics for the mouth. You can become a professional retail partner today by visiting our WEBSITE. Still looking for more info about ProBiora? Request a lunch and learn by filling out the info HERE.
1 Deo PN, Deshmukh R. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol. 2019 Jan-Apr;23(1):122-128. doi: 10.4103/jomfp.JOMFP_304_18. PMID: 31110428; PMCID: PMC6503789.
2 Peng X, Cheng L, You Y, Tang C, Ren B, Li Y, Xu X, Zhou X. Oral microbiota in human systematic diseases. Int J Oral Sci. 2022 Mar 2;14(1):14. doi: 10.1038/s41368-022-00163-7. PMID: 35236828; PMCID: PMC8891310.
3 Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. Journal of Dental Research. 2012;91(10):914-920. doi:10.1177/0022034512457373
4 Sudhakara P, Gupta A, Bhardwaj A, Wilson A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dentistry Journal. 2018; 6(2):10. https://doi.org/10.3390/dj6020010
5 Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012 Sep;91(9):816-20. doi: 10.1177/0022034512453589. Epub 2012 Jul 6. PMID: 22772362; PMCID: PMC3420389.
6 Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012 Sep;91(9):816-20. doi: 10.1177/0022034512453589. Epub 2012 Jul 6. PMID: 22772362; PMCID: PMC3420389.
7 Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011 Nov 17;10(5):497-506. doi: 10.1016/j.chom.2011.10.006. Epub 2011 Oct 27. PMID: 22036469; PMCID: PMC3221781.
8 Mei F, Xie M, Huang X, Long Y, Lu X, Wang X, Chen L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens. 2020; 9(11):944. https://doi.org/10.3390/pathogens9110944
9 Kamer, AR, Pushalkar, S, Gulivindala, D, et al. Periodontal dysbiosis associates with reduced CSF Aβ42 in cognitively normal elderly. Alzheimer’s Dement. 2021; 13:e12172. https://doi.org/10.1002/dad2.12172
10 Borsa L, Dubois M, Sacco G, Lupi L. Analysis the link between periodontal diseases and Alzheimer’s disease: a systematic review. Int J Environ Res Public Health. 2021;18(17):9312. doi:3390/ijerph18179312
11 Kriebel K, Hieke C, Müller-Hilke B, Nakata M, Kreikemeyer B. Oral biofilms from symbiotic to pathogenic interactions and associated disease–connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front Microbiol.2018;9:53. doi:3389/fmicb.2018.00053
12 Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5:eaau3333. doi: 10.1126/sciadv.aau3333
13 Ishida, N., Ishihara, Y., Ishida, K., Tada, H., Funaki-Kato, Y., Hagiwara, M., et al. (2017). Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech. Dis. 3:15. doi: 10.1038/s41514-017-0015-x
14 Asher, S, Stephen, R, Mäntylä, P, Suominen, AL, Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J Am Geriatr Soc. 2022; 70( 9): 2695- 2709. doi:10.1111/jgs.17978
15 Gao L, Shuai Y, Wen L, Zhang H, Zhang Y, Zhang X. Benefit and safety of antibiotics for Alzheimer’s disease: Protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2022 Nov 25;101(47):e31637. doi: 10.1097/MD.0000000000031637. PMID: 36451430; PMCID: PMC970486
16 Rowisnka I, Szyperska-Slaska A, Zariczny P, Paslawski R, Kramkowski K, Kowalczyk P. The influence of diet on oxidative stress and inflammation induced by bacterial biofilms in the human oral cavity. Materials (Basel). 2021;14(6):1444. doi:3390/ma14061444
17 Vanhatalo A, L’Heureux JE, Kelly J, et al. Network analysis of nitrate-sensitive oral microbiome reveals interactions with cognitive function and cardiovascular health across dietary interventions. Redox Biol. 2021;41:101933. doi:1016/j.redox.2021.101933
18 Olsen I, Potempa J. Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases. J Oral Microbiol. 2014;6. doi:3402/jom.v6.24800
19 Sánchez MC, Ribeiro-Vidal H, Esteban-Fernández A, et al. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement Altern Med. 2019;19(1):145.
20 Morales A, Gandolfo A, Bravo J, et al. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: a randomized placebo-controlled trial with 9-month follow-up. J Appl Oral Sci. 2018;26:e20170075. doi:1590/1678-7757-2017-0075
21 Ishikawa KH, Mita D, Kawamoto D, et al.Probiotics alter biofilm formation and the transcription of Porphyromonas gingivalis virulence-associated genes. J Oral Microbiol. 2020;12(1):18055553. doi:1080/20002297.2020.1805553
22 Jansen PM, Abdelbary MMH, Conrads G. A concerted probiotic activity to inhibit periodontitis-associated bacteria. PLoS One. 2021;16(3):e0248308. doi:1371/journal.pone.0248308
23 Matsubara VH, Bandara HM, Ishikawa KH, Mayer MP, Samaranayake LP. The role of probiotic bacteria in managing periodontal disease: a systematic review. Expert Rev Anti Infect Ther. 2016;14(7):643-655. doi:1080/14787210.2016.1194198
24 Radaic A, Ye C, Parks B, et al.Modulation of pathogenic oral biofilms towards health with nisin probiotic. J Oral Microbiol. 2020;12(1):1809302. doi:1080/20002297.2020.1809302
25 MacDonald KW, Chanyi RM, Macklaim JM, Cadieux PA, Reid G, Burton JP. Streptococcus salivarius inhibits immune activation by periodontal disease pathogens. BMC Oral Health. 2021;21(1):245. doi:1186/s12903-021-01606-z
26 Allaker RP, Stephen AS. Use of probiotics and oral health. Curr Oral Health Rep. 2017;4(4):309-318. doi:1007/s40496-017-0159-6
27 Morales A, Gandolfo A, Bravo J, Carvajal P, Silva N, Godoy C, Garcia-Sesnich J, Hoare A, Diaz P, Gamonal J. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: a randomized placebo- controlled trial with 9-month follow-up. J Appl Oral Sci. 2018 Jan 18;26:e20170075. doi: 10.1590/1678-7757-2017-0075. PMID: 29364340; PMCID: PMC5777419.
28 Saïz P, Taveira N, Alves R. Probiotics in Oral Health and Disease: A Systematic Review. Applied Sciences. 2021; 11(17):8070. https://doi.org/10.3390/app11178070
29 Penala S, Kalakonda B, Pathakota KR, Jayakumar A, Koppolu P, Lakshmi BV, Pandey R, Mishra A. Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: A randomized controlled trial. J Res Pharm Pract 2016;5:86-93